In 2018, FDA licensed Vaxelis for use in children age 6 weeks through 4 years: it is indicated as a 3-dose series for infants at ages 2, 4, and 6 months. ACIP voted to add Vaxelis to the Vaccines for Children (VFC) Program in The MSP Vaccine Company was created as a joint venture between Merck and Sanofi Pasteur to produce Vaxelis.
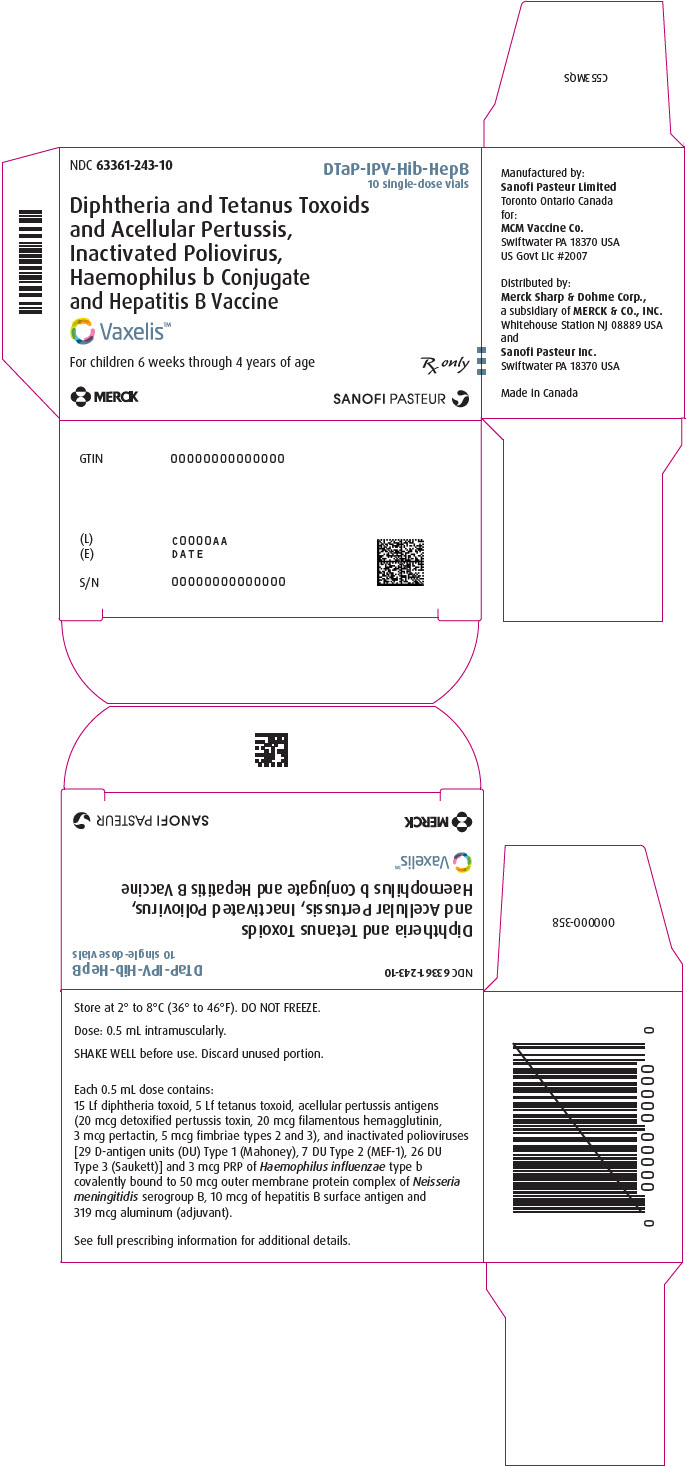
Vaxelis (DTaP-IPV-Hib-HepB) Fact Sheet Vaxelis is a hexavalent combination vaccine approved to prevent diphtheria, tetanus, pertussis, polio, Haemophilus influenzae type b, and hepatitis B. CS324755-A Storage Preparation. Administration; Between 2°C to 8°C (36°F to 46°F)
VAXELIS is a vaccine given to protect your child from getting diphtheria, tetanus (lockjaw), pertussis (whooping cough), polio, Hib
Do not combine VAXELIS through reconstitution or mix with any other vaccine. Storage information for VAXELIS Here are some key things to keep in mind when storing VAXELIS: VAXELIS is supplied in a single-dose vial in packages of 10 vials or in a single-dose, prefilled syringe in packages of 10 syringes Store at 2°C to 8°C (36°F to 46°F)
Vaxelis is the first and only hexavalent (six-in-one) combination vaccine available in the U.S. Vaxelis is a vaccine given to protect your child from getting diphtheria, tetanus (lockjaw), pertussis (whooping cough), polio, Hib (Haemophilus influenzae type b), and hepatitis B. Your child cannot get any of these diseases from Vaxelis.
Vaxelis (DTaP-IPV-Hib-HepB) Fact Sheet Vaxelis is a hexavalent combination vaccine approved to prevent diphtheria, tetanus, pertussis, polio, Haemophilus influenzae type b, and hepatitis B. CS324755-A Storage Preparation. Administration; Between 2°C to 8°C (36°F to 46°F) VAXELIS is a vaccine given to protect your child from getting diphtheria, tetanus (lockjaw), pertussis (whooping cough), polio, Hib Do not combine VAXELIS through reconstitution or mix with any other vaccine. Storage information for VAXELIS Here are some key things to keep in mind when storing VAXELIS: VAXELIS is supplied in a single-dose vial in packages of 10 vials or in a single-dose, prefilled syringe in packages of 10 syringes Store at 2°C to 8°C (36°F to 46°F) Vaxelis is the first and only hexavalent (six-in-one) combination vaccine available in the U.S. Vaxelis is a vaccine given to protect your child from getting diphtheria, tetanus (lockjaw), pertussis (whooping cough), polio, Hib (Haemophilus influenzae type b), and hepatitis B.
Your child cannot get any of these diseases from Vaxelis.
VAXELIS is to be administered as a 3-dose series at 2, 4, and 6 months of age. The first dose may be given as early as 6 weeks of age. Three doses of VAXELIS constitute a primary immunization course against diphtheria, tetanus, H. influenzae type b invasive disease and poliomyelitis. VAXELIS may be used to complete the hepatitis B immunization
Forme et Présentation. Vaxelis suspension injectable en seringue préremplie. Suspension blanche à blanchâtre, trouble, homogène. Vaccin diphtérique, tétanique, coquelucheux (acellulaire, multicomposé), de l'hépatite B (ADNr), poliomyélitique (inactivé) et conjugué de l'Haemophilus influenzae type b, adsorbé. Nature et contenu de l'emballage extérieur
VAXELIS is a vaccine indicated for active immunization to prevent diphtheria, tetanus, pertussis, poliomyelitis, hepatitis B, and invasive disease due to Haemophilus influenzae type b. VAXELIS is approved for use as a 3-dose series in children 6 weeks through 4 years of age (prior to the 5th birthday). Important Safety Information for VAXELIS
Indication for VAXELIS VAXELIS is a vaccine indicated for active immunization to prevent diphtheria, tetanus, pertussis, poliomyelitis, hepatitis B, and invasive disease due to Haemophilus influenzae type b. VAXELIS is approved for use as a 3-dose series in children 6 weeks through 4 years of age (prior to the 5th birthday).
FDA Approves VAXELIS™, Sanofi and Merck's Pediatric Hexavalent
VAXELIS is to be administered as a 3-dose series at 2, 4, and 6 months of age. The first dose may be given as early as 6 weeks of age. Three doses of VAXELIS constitute a primary immunization course against diphtheria, tetanus, H. influenzae type b invasive disease and poliomyelitis. VAXELIS may be used to complete the hepatitis B immunization Forme et Présentation.
Vaxelis suspension injectable en seringue préremplie. Suspension blanche à blanchâtre, trouble, homogène. Vaccin diphtérique, tétanique, coquelucheux (acellulaire, multicomposé), de l'hépatite B (ADNr), poliomyélitique (inactivé) et conjugué de l'Haemophilus influenzae type b, adsorbé. Nature et contenu de l'emballage extérieur VAXELIS is a vaccine indicated for active immunization to prevent diphtheria, tetanus, pertussis, poliomyelitis, hepatitis B, and invasive disease due to Haemophilus influenzae type b. VAXELIS is approved for use as a 3-dose series in children 6 weeks through 4 years of age (prior to the 5th birthday).
Important Safety Information for VAXELIS Indication for VAXELIS VAXELIS is a vaccine indicated for active immunization to prevent diphtheria, tetanus, pertussis, poliomyelitis, hepatitis B, and invasive disease due to Haemophilus influenzae type b. VAXELIS is approved for use as a 3-dose series in children 6 weeks through 4 years of age (prior to the 5th birthday).
Find billing codes for VAXELIS® (Diphtheria and Tetanus Toxoids and Acellular Pertussis, Inactivated Poliovirus, Haemophilus b Conjugate and Hepatitis B Vaccine), including CPT, NDC, and ICD-10 codes.
1 VAXELIS ™ is the result of the U.S.-based business joint-partnership (MCM) created in 1991 between Merck & Co., Inc., Connaught Laboratories and Pasteur Mérieux Serums & Vaccins, the latter two now known as Sanofi Pasteur, the vaccines unit of Sanofi Media Contacts: Pamela Eisele, (267) 305-3558 Kristen Drake, (908) 334-Investor Contacts: Teri Loxam, (908) 740-1986
A 3-dose series of VAXELIS does not constitute a primary immunization series against pertussis; an additional dose of pertussis-containing vaccine is needed to complete the primary series. Before administering VAXELIS, please read the accompanying Prescribing Information. The Patient Information also is available.
Patient Information VAXELIS (pronounced vak-sel-lis) Pati
PDF Patient Information VAXELIS (pronounced vak-sel-lis) Pati
Find billing codes for VAXELIS® (Diphtheria and Tetanus Toxoids and Acellular Pertussis, Inactivated Poliovirus, Haemophilus b Conjugate and Hepatitis B Vaccine), including CPT, NDC, and ICD-10 codes. 1 VAXELIS ™ is the result of the U.S.-based business joint-partnership (MCM) created in 1991 between Merck & Co., Inc., Connaught Laboratories and Pasteur Mérieux Serums & Vaccins, the latter two now known as Sanofi Pasteur, the vaccines unit of Sanofi Media Contacts: Pamela Eisele, (267) 305-3558 Kristen Drake, (908) 334-Investor Contacts: Teri Loxam, (908) 740-1986 A 3-dose series of VAXELIS does not constitute a primary immunization series against pertussis; an additional dose of pertussis-containing vaccine is needed to complete the primary series. Before administering VAXELIS, please read the accompanying Prescribing Information. The Patient Information also is available. Patient Information VAXELIS (pronounced vak-sel-lis) Pati
Download multi-vaccines VISs (DTaP, Hib, Hep B, Polio and PCV) in English and Spanish, plus other languages. PDF format.
VAXELIS is to be administered as a 3-dose series at 2, 4, and 6 months of age. The first dose may be given as early as 6 weeks of age. Three doses of VAXELIS constitute a primary immunization course against diphtheria, tetanus, H. influenzae type b invasive disease and poliomyelitis. VAXELIS may be used to complete the hepatitis B immunization
3 VAXELIS may be used to complete the hepatitis B vaccination series following 1 or 2 doses of other hepatitis B vaccines, in infants and children born of HBsAg-negative mothers and who are
DTaP-IPV-Hib-HepB Vaxelis 2, 4, 6 months *** *Recommended vaccine for American Indian/Alaska Native children **Hib component of Pentacel is PRP-T. Hib component of Vaxelis is PRP-OMP. ***Vaxelis is not recommended for the booster dose. A different Hib-containing vaccine should be administered as a booster at 12-15 months. 3
PDF Evidence to Recommendations and Proposed Recommendations: Use of
Download multi-vaccines VISs (DTaP, Hib, Hep B, Polio and PCV) in English and Spanish, plus other languages. PDF format. VAXELIS is to be administered as a 3-dose series at 2, 4, and 6 months of age. The first dose may be given as early as 6 weeks of age. Three doses of VAXELIS constitute a primary immunization course against diphtheria, tetanus, H.
influenzae type b invasive disease and poliomyelitis. VAXELIS may be used to complete the hepatitis B immunization 3 VAXELIS may be used to complete the hepatitis B vaccination series following 1 or 2 doses of other hepatitis B vaccines, in infants and children born of HBsAg-negative mothers and who are DTaP-IPV-Hib-HepB Vaxelis 2, 4, 6 months *** *Recommended vaccine for American Indian/Alaska Native children **Hib component of Pentacel is PRP-T. Hib component of Vaxelis is PRP-OMP. ***Vaxelis is not recommended for the booster dose. A different Hib-containing vaccine should be administered as a booster at 12-15 months.
3
Vaxelis is a vaccine containing active substances derived from diphtheria, tetanus, pertussis and Haemophilus influenzae type-b bacteria, the hepatitis B virus, and inactivated polioviruses. It is used in babies and toddlers aged from six weeks to protect against the following infectious diseases:
A 3-dose series of VAXELIS does not constitute a primary immunization series against pertussis; an additional dose of pertussis-containing vaccine is needed to complete the primary series. Before administering VAXELIS, please read the accompanying Prescribing Information. The Patient Information also is available.
A 3-dose series of VAXELIS does not constitute a primary immunization series against pertussis; an additional dose of pertussis-containing vaccine is needed to complete the primary series. Before administering VAXELIS, please read the accompanying Prescribing Information. The Patient Information also is available.
Before administering VAXELIS, please read the accompanying Prescribing Information. The Patient Information also is available. Do not administer VAXELIS to anyone with a history of severe allergic reaction to a previous dose of VAXELIS, any ingredient of VAXELIS, or any other diphtheria toxoid,
Vaccine Components of VAXELIS® (Diphtheria and Tetanus Toxoids and
Vaxelis is a vaccine containing active substances derived from diphtheria, tetanus, pertussis and Haemophilus influenzae type-b bacteria, the hepatitis B virus, and inactivated polioviruses. It is used in babies and toddlers aged from six weeks to protect against the following infectious diseases: A 3-dose series of VAXELIS does not constitute a primary immunization series against pertussis; an additional dose of pertussis-containing vaccine is needed to complete the primary series. Before administering VAXELIS, please read the accompanying Prescribing Information. The Patient Information also is available. A 3-dose series of VAXELIS does not constitute a primary immunization series against pertussis; an additional dose of pertussis-containing vaccine is needed to complete the primary series.
Before administering VAXELIS, please read the accompanying Prescribing Information. The Patient Information also is available. Before administering VAXELIS, please read the accompanying Prescribing Information. The Patient Information also is available. Do not administer VAXELIS to anyone with a history of severe allergic reaction to a previous dose of VAXELIS, any ingredient of VAXELIS, or any other diphtheria toxoid,
Other. Vaxelis® (Diphtheria and Tetanus Toxoids and Acellular Pertussis, Inactivated Poliovirus, Haemophilus b Conjugate and Hepatitis B Vaccine)
Vaxelis™ is an appealing option because of its simplicity as a combination vaccine. The 2-, 4-, and 6-month doses of PEDIARIX ™ (DTap, Hep B, IPV) could be replaced by Vaxelis™. The 2-and 4-month doses of PedvaxHIB® in the AI/AN routine childhood immunization schedule would be obsolete. The use of Vaxelis™ would save
VAXELIS is a vaccine indicated for active immunization to prevent diphtheria, tetanus, pertussis, poliomyelitis, hepatitis B, and invasive disease due to Haemophilus influenzae type b (Hib). VAXELIS is approved for use as a 3-dose series in children 6 weeks through 4 years of age (prior to the 5 th birthday).
VAXELIS is a vaccine indicated for active immunization to prevent diphtheria, tetanus, pertussis, poliomyelitis, hepatitis B, and invasive disease due to Haemophilus influenzae type b. VAXELIS is approved for use as a 3-dose series in children from 6 weeks through 4 years of age (prior to the 5 th birthday). ()
Vaxelis: Package Insert / Prescribing Information - Drugs.com
Other. Vaxelis® (Diphtheria and Tetanus Toxoids and Acellular Pertussis, Inactivated Poliovirus, Haemophilus b Conjugate and Hepatitis B Vaccine) Vaxelis™ is an appealing option because of its simplicity as a combination vaccine. The 2-, 4-, and 6-month doses of PEDIARIX ™ (DTap, Hep B, IPV) could be replaced by Vaxelis™. The 2-and 4-month doses of PedvaxHIB® in the AI/AN routine childhood immunization schedule would be obsolete. The use of Vaxelis™ would save VAXELIS is a vaccine indicated for active immunization to prevent diphtheria, tetanus, pertussis, poliomyelitis, hepatitis B, and invasive disease due to Haemophilus influenzae type b (Hib).
VAXELIS is approved for use as a 3-dose series in children 6 weeks through 4 years of age (prior to the 5 th birthday). VAXELIS is a vaccine indicated for active immunization to prevent diphtheria, tetanus, pertussis, poliomyelitis, hepatitis B, and invasive disease due to Haemophilus influenzae type b. VAXELIS is approved for use as a 3-dose series in children from 6 weeks through 4 years of age (prior to the 5 th birthday). ()